by Philip Remedios | CEO, Director of Design & Development
All societies around the world have experienced substantial changes since the onset of COVID-19 with lifestyles displaced and corporate cultures newly embracing a work-from-home concept with the help of the Internet of Things (IoT) and highly evolved, online connectivity tools. A decentralized workplace is becoming more common than the centralized (work in the office) norm, including the trend towards people managing their health. Although idealism behind home-based or distance-care constructs have been around for some time, the growing attention they are receiving is primarily due to the focus on decreasing operating costs, as opposed to supporting patient-driven requests. Due to medical practices impacted by the pandemic, out-of-hospital consultation and remote diagnostics have taken center stage in an attempt to mitigate risk from viral transmission and lessen hospital burdens. This has also increased the importance of a multi-advantageous workflow that encompasses less clinical overhead, higher efficiencies, faster transactions and greater convenience for all parties.
When we add the changes to workflow due to COVID-related events to the last decade’s evolution towards overhead-reduction through distance care, it is apparent how quickly changes to the medical device environment are occurring. This article uncovers key aspects of the more recent evolution in medical device design and illustrates how the move towards more home-based and distance-care may further evolve in a post-COVID environment.
Design Innovation Evolution
More than a decade ago, medical device innovation focused mostly on advancing capability, accuracy and convenience. Capital equipment became complicated and expensive with every iteration, along with pre-packaged single-use disposables that even included integrated electronics. Due to these increasing costs, more fiscally conscious emerging markets negated this costly new trend, along with some European nations, which were trying to minimize product waste by maximizing recycling programs. Instead, they sought to adopt a better approach. Therefore, designers focused on concepts that comprised reusable components and devices to enable business expansion in cost-sensitive markets.
Design requirements have noticeably changed within the last 10 years. Originally, emerging markets sought simply utilizing lower-priced designs—just the essentials as opposed to first-world devices that deliver all the “bells and whistles.” Rising healthcare costs (primarily in the United States) drove the need for change. This caused the trend towards “lower cost” versions of legacy devices from emerging international markets before bringing them back onshore, where even at times a half-generation iteration could be optimized to use for domestic market needs.
Innovative design was progressively being boiled down to maintaining and typically reducing “cutting-edge” functionality to achieve an overall lower per-case cost. Wholesalers would be requested to cut overhead costs by 30–50% or more as part of the new norm. Any deployed technology, materials, procedure, reusability and even sterilization techniques would be scrutinized and evaluated for further cost streamlining activities. Optimized usability, a critical component of good design practices (GDP), use-safety and ease-of-use attributes must also be included without cost impact.
Many cost-cutting measures that involve operational efficiency and maximized procedural success can be vital, especially considering, for example, that operating room time can cost $100 per minute and reducing procedural costs by 20% or 30% can translate to substantial savings. In fact, it can enable an institution to increase its profit margins by performing more operations per day. Moreover, if a design invokes a greater opportunity for procedural success by reducing use-error and therefore impacts revisional action (which is usually not reimbursable), the hospital benefits by achieving additional operational cost savings.
Prioritization of Costs and Further Mitigating Risks
In a concentrated effort to decrease total cost, there is an evolving trend regarding who uses the medical device. For instance, devices traditionally managed by physicians and/or surgeons are now operated by lower-paid clinical fellows, nurses, and medical technicians. User interfaces are made to be simple to use and much more intuitive than in the past so that they could be operated more easily by less experienced staff. Therefore, developing varying levels of interface operability to accommodate the range of ability and training of the user is a key consideration. Additionally, varied parameters should be customized where doctors could set and lock the limits on procedural protocols if it is warranted. Med-techs can access everyday setup and operations controls and nurses can supervise and monitor only critical parameters during their shifts in order to minimize mental fatigue.
These evolving use-requirements play a role in recent device designs. For instance, with capital equipment, mobility is essential among intermittently used devices that are shared between operating rooms, wards and other points-of-care (POC) in order to decrease capital expenditure costs. Permanent-residing devices should leverage shared storage systems and be attached to off-the-floor gantries to avoid any potential accidents due to cable/wire trip hazards and since floor space is typically at a premium.
Equipping shelf or rack-mounting with the user interface located on the front face is beneficial. While this may not appear to be a prominent design challenge, it needs to be considered for many devices that require medical grade single-use couplings as used with peristaltic pumps, filters, connections, and sensors, which typically do not provide a lot of room for a modest-sized GUI (graphical user interface) and hard-keys. Adhering to the use of standardized software protocols enables many devices to display data on a shared monitor, which makes for a better user experience (UX). Both current and trending use-cases serve as great examples, which can help the design team to better conceptualize a more suitable system architecture to meet real-world needs for the useful life of the device.
The Emerging New End User is the Patient
Patients today expect to be more involved in their healthcare, as this ongoing trend is a result of a proliferation of in-home and wearable devices. Initially diagnostic in nature, these devices were developed to assist providers in monitoring critically ill patients’ vital signs daily. Thanks to the launch of digital technology and the interconnected society it created, secure data can be collected, assessed and shared with relative ease. Wearables such as pumps, drug-delivery patches, blood pressure cuffs as well as several countertop devices have become commonplace and covered under insurance plans, which enables greater adoption. The industry as a whole is capitalizing on this new interoperable paradigm shift, which is truly advancing healthcare. Designers’ perspectives have evolved as they have started to consider these patient-operated devices as consumer products and in need of the same sleek aesthetics and ease-of-use as the smartphone or fancy kitchen appliances.
The patented concept in the images in Table I illustrates how a global design platform can be customized to adapt to a myriad of market and context-driven installation and UX requirements. Once the base housing, designed around the electronic controller and power system with modular side caps and front display module is properly outfitted, the internal software can be factory configured to suit a wide array of market drivers including cost, functionality, feature-sets and environmental workflows.

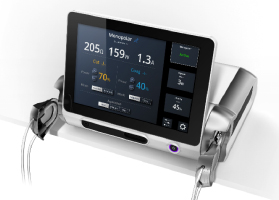

Cost-reducing innovation can best be leveraged by understanding the device’s entire daily workflow and identifying upfront all areas of opportunity. For example, requirements for maintenance and handling can impact overhead costs. Storage space efficiency is another example that can enhance the marketable value of a new design (see Image 1).

Hybrid Instrument Design: Durable and Disposable
Today, the industry is shifting away from 100% disposable strategies. By reposing non-wearing, costly portions of the device (re-sterilization requirements and costs must of course be added to the design requirements and per-case cost), the sub-cost can be amortized over hundreds of cases, which over time can vastly reduce the adjusted unit cost. Electronics, motors, sensors, and optics often embedded in these sub-assemblies must withstand the selected re-sterilization protocol, which may involve high-heat, moisture, gas flow, aeration and/or material durability. Depending on the market, requirements could necessitate reposable parts to be sterilized using hospital-operated systems, often steam-based. It is recommended to check with the FDA’s published guidance document, “Reprocessing Medical Devices in Health Care Settings: Validation Methods and Labeling”.
The highly scrutinized disposable components for single-use function, which are difficult to re-sterilize, often include such mechanical parts as needles, cannulas, cutting blades, springs, wearable electrodes, plus considerations may need to be made for part geometries and materials. All other durable components utilized help to make up “hybrid” devices. They are easily partitioned and (dis)assembled into reusable parts and easily cleaned and re-used. As a general rule of thumb: the longer the mean-time-before-failure (MTBF), the greater the cost savings achieved with the amortized per-case cost of the device.
Critical Strategy Considerations for Concurrent Product Development
What usually comes with greater technical complexity is increased project risk, such as when the launch of the device is invariably lengthened, and the time-to-market stalled. A tried-and-true marketing strategy should define first and foremost the requirements for a minimally viable product (MVP) and develop it as the Gen1 device. Keep in mind that the underlying MVP design architecture should be intentionally developed to provide a robust and upgradeable platform to spawn a series of version updates over its life, either for the same or for other markets. For those complex devices created for a long service life, integrating a modular approach not only makes localized swap-outs for newer, sleeker, more cost-effective replacements easier but also help to acknowledge which components (e.g., displays, controllers, data storage and power systems) will become obsolete.
For example, one medical device maker used this “pipeline-approach” to develop and launch a low-cost MVP Gen1 device for its targeted international market (China and India) while concurrently advancing configurations and using US/EU market modular upgrades to their platform. Such an approach allowed for subsequent and risker iterations of the device to be launched into more lucrative first world markets at higher, sustainable cost-points. Achieving a staged global release provided them with actual operational performance, reliability and clinical data from their initial MVP offering and optimized their Gen2++ devices before they went to market. Applying this strategy additionally gave them a competitive edge in which the competition was faced with a moving target over time, making it much more difficult for them to leapfrog.
The Future Outlook
Globally, the medical device industry continues to strive to achieve cost savings across the board—whether it is through CapEx, per-case costs, and/or maximizing operational efficiencies all while minimizing cost-inducing use-error. Modern innovation teams have come to realize that they need to be far more practical in balancing real-market drivers with device technology. They know cost savings require a clear understanding of the competitive landscape, cost limits and knowledge of which market-penetration strategies to use. Design engineers know that adopting macroscopic visibility into these project goals is critical to enable the actual realization of the project mission. No longer can the traditional desire to maximize absolute capability be the overriding endgame, especially as increasing more stakeholders scrutinize value-added features and functions against cost-creep and project risk.
Despite the undisputed call for cost control, medical technology will continue to advance because functional capability and positive patient outcomes will always be the most important competitive differentiator. In our fast-maturing and intensely competitive industry, successful design engineers wear many hats in order to fully understand and balance the true value proposition within today’s fast evolving med-device world.



