Today’s necessity to reduce healthcare costs is generating a greater demand for medical electronics equipment that improves and expands patient diagnostics inside and outside healthcare facilities. For instance, portable medical instruments such as glucose meters, blood pressure monitors, oxygen meters, and automated external defibrillators (AED) have undergone many design considerations to be developed for doctors, paramedics, public use, and at home with at-risk patients. This article delves into the design considerations, challenges, and regulatory aspects of medical electronics and provides a case study involving automated external defibrillators (AEDs).

Enhancing Electronic Medical Device Capabilities
Most medical devices for use by the public or at home are being designed with communication capabilities to provide continuous information to caregivers almost anywhere, which can further help decrease healthcare costs. Handheld devices are also improving various diagnostic procedures in medical offices and for at-home us
Wearable electronic medical devices for at-home use are expanding in the breadth of applications. For instance, a wearable electrocardiogram (ECG) device can detect atrial fibrillation through the electrode pads. Designed with communication capabilities, these ECG devices send readings to the patient’s doctor. While these electronic medical devices hold the potential to deliver better-quality care, they require a lot of user research in the initial design process. Interactive discovery methods identify latent user needs and characterize usage scenarios such as at-home use, in public or by an EMT or practitioner. When conducting the discovery phase with the design process, it requires a team of analytical researchers and designers knowledgeable in the usage environment (either contextual inquiry or ethnography).
Compliance
Bringing a new electronic medical device of any kind through all the steps for FDA approval is a complex and very time-intensive process. Making sure design engineers and the R&D team take all practical steps to shorten the product development cycle often requires time and resource investments in the conceptual design process, including usability testing.
Medical devices need to meet a complex set of safety requirements. Compliance regulations can substantially affect the design, including mechanical, electrical, and graphical user interface (GUI). The use of empirical methodology, one of the foundational building blocks of good usability engineering, establishes testable usability inputs for downstream formative studies, culminating in an FDA-required human factors engineering report.
Medical device compliance also requires unique disciplines in the design to meet approvals from a notified body, such as UL, TUV, Intertek, or any other of the internationally recognized agencies while the FDA requires strict adherence to IEC guidance documents.
Collateral standards (numbered 60601-1-X) publish requirements for essential safety and performance, for example, Electromagnetic Disturbances (IEC 60601-1-2). Particular standards are published with requirements for specific types of medical devices. Designing medical devices that incorporate electronics can become complicated due to the maze of regulations. Observation of processes in the IEC-60601 documents is instrumental in becoming compliant.
A Case for Discussion: Automated External Defibrillators
There are key requirements for the basic safety and essential performance of electrocardiographs and cardiac defibrillators, for instance, the spacings for high voltage traces (referred to as creep-age and clearance) must be maintained. RF emissions and susceptibility must be verified and tested to ensure that the electromagnetic compatibility (EMC) requirements of the collateral standard IEC 60601-1-2 are met.
The challenges of designing portable medical devices involve reducing weight and size while continuing to meet the requirements of a complex therapy. In this use case, the study examines a portable AED for use in airports, schools, and other public settings. Due to the use by a novice in emergency situations, it is critical to simplify the interface, instructions, and operations, which are all key considerations in the design process.
In the case of the AED, Figure 1 depicts the electrical subsystems required to detect and analyze ECG waveforms and deliver up to a 2000 V shock to the patient when required.
Some of the components are significant in size, such as the battery and the capacitor required to charge, store, and deliver up to a 220 Joule pulse to the patient’s chest and heart. Modern lithium batteries help reduce weight, but battery capacity and size are always a compromise. Miniaturized packaging must allow close proximity of highly sensitive analog circuits amplifying infinitely weak signals from the patient’s heart. A massive jolt of energy must be delivered in synchronized timing to safely bring a patient’s heart back to a normal beat.
Today’s modern devices can meet the stringent requirements for EMC in medical devices, but the overall system design becomes more challenging. Plastic enclosures allow form-fitting, colored, and textured surfaces to idealize the interfaces to the user. Adding metal coatings inside plastics often becomes complex and cost prohibitive. Typically, a traditional metal enclosure that provides Faraday shielding is no longer available to block electromagnetic fields.Multi-layer printed circuit boards (PCB) can be designed to contain emissions and be immune to susceptibility from external RF challenges. Cabling needs to be minimized and interfaces must be protected and filtered from noise to be successful in meeting these challenges. Connectivity by wireless communication is oftentimes required and needs to be an integral part of the overall design to ensure antenna position is optimal and that EMC test results are not compromised.
In designing portable public-space AEDs, preventing accidental shocks to individuals who don’t require them is a critical requirement. Ensuring ease of operation in emergencies with a straightforward power button that triggers voice prompts is pivotal. AEDs need to provide both audio and visual cues throughout the process to reinforce user guidance.
Audio and Visual Prompting Design Considerations
Besides voice instructions, AEDs use other sounds to help users. There is a sound that specifies a shock is indicated, and that the AED is “charging” up to deliver a shock. Another distinct sound alerts the user that the AED is ready to deliver a shock. These sounds coincide with specific voice instructions such as “shock indicated” and “press the shock button.”
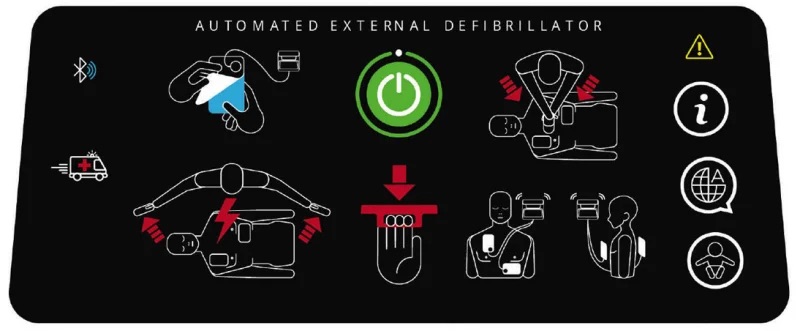
As shown in the graphic in Figure 2, there is visual prompting that flashes colored lights indicating that the power is on. Other lights flash to let the user know an analysis is being done as well as which button to push to deliver the shock. Both the audio and visual instructions coincide to assist the novice user through the entire process from start to finish.
Conclusion
Also, correct pad placement is important for the effective delivery of energy through to the heart. Usability studies indicate that there are users who did not remove the clothes covering the chest and instead adhered the pads to the clothing. Other users did not peel the protective backing off the pads. This causes problems because the pads must be affixed to the bare skin.
This is only one example of countless potential scenarios that could occur regarding the risks from novice users improperly operating an electronic medical device.
Experienced design teams that have the expertise in the regulatory aspect of device design during the R&D process (involving UX/UI design, human factors engineering, user research, prototyping) will be coveted, especially with the growth that is occurring in the home medical equipment market, which was valued at US $12.23 billion in 2021 and projected to reach US $21.13 billion by 2030. 2
Couple this growth with the shortage of skilled R&D staff, expect many more device manufacturers to rely on consultancies to direct their design processes, especially compliance. With this growth and an expected shortage of skilled R&D staff, many more medical device developers can expect to rely on experienced consultants to help support their design and compliance processes.
References
- IEC 60601-1:2023, International Electrotechnical Commission, https://www.iec.ch.
- “U.S. Home Medical Equipment Market Size to Surpass US$ 21.13 BN By 2030,” BioSpace, July 12, 2022.
Originally posted on Medical Design Briefs. December 21, 2023.



