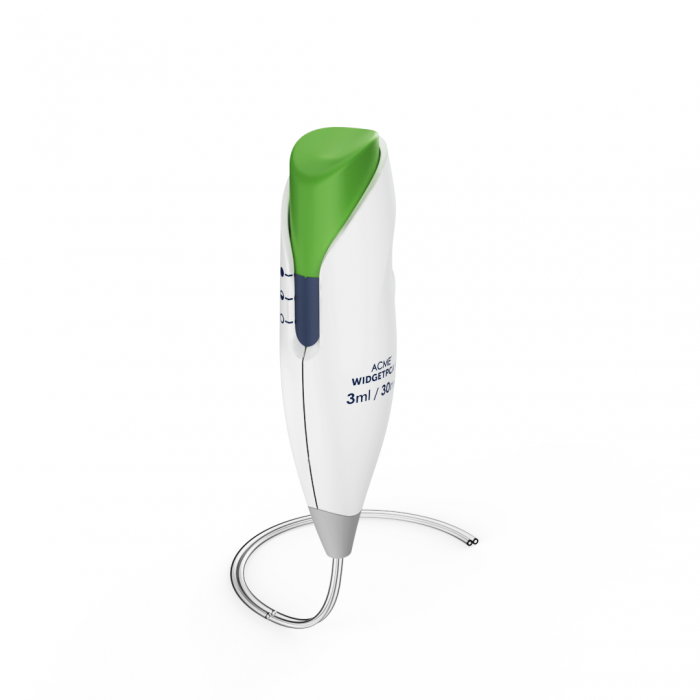
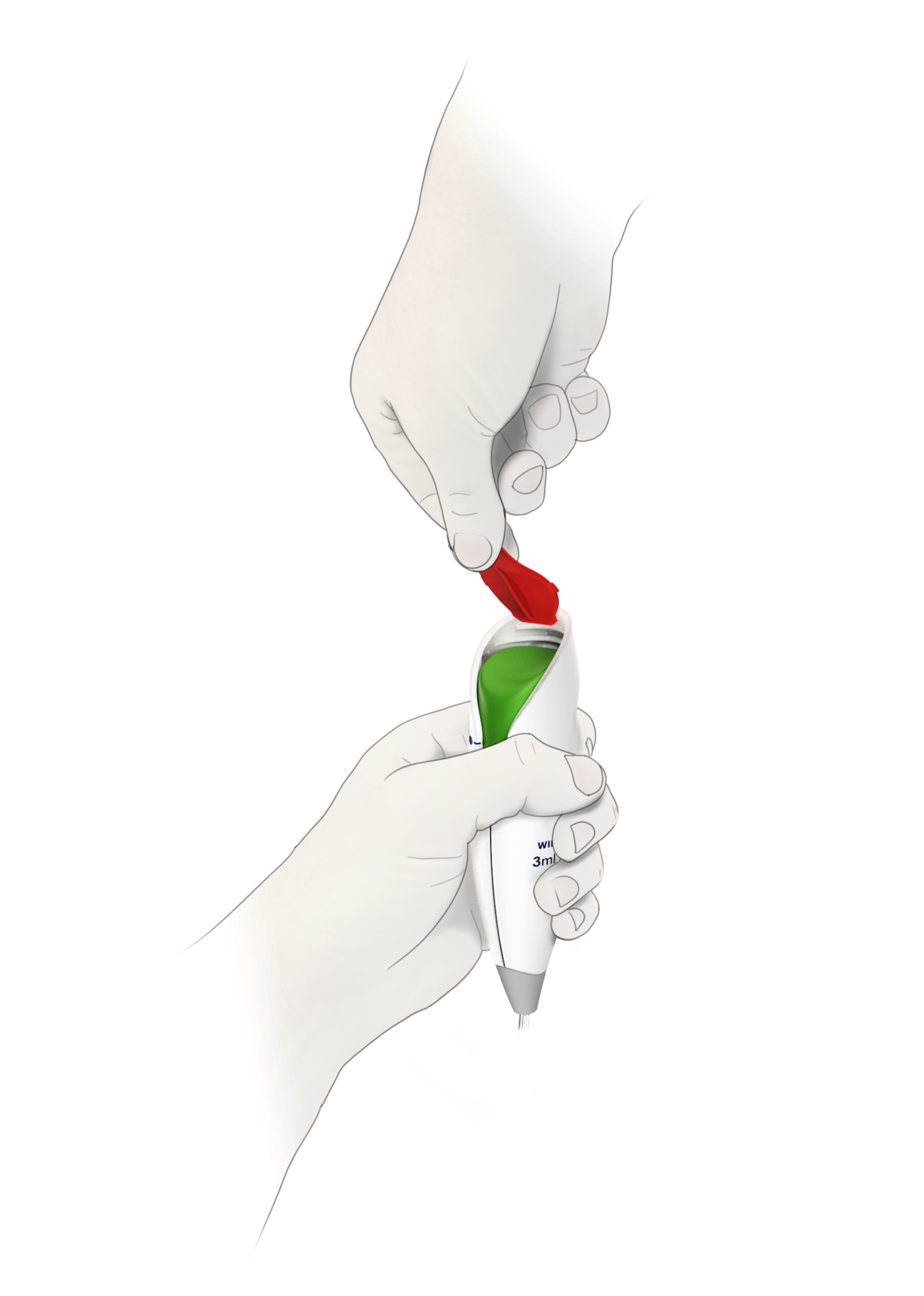
ON-Q* with Bolus Pump Infusion Device
Designed for Comfort, Simplicity, and Safe At-Home Pain Relief
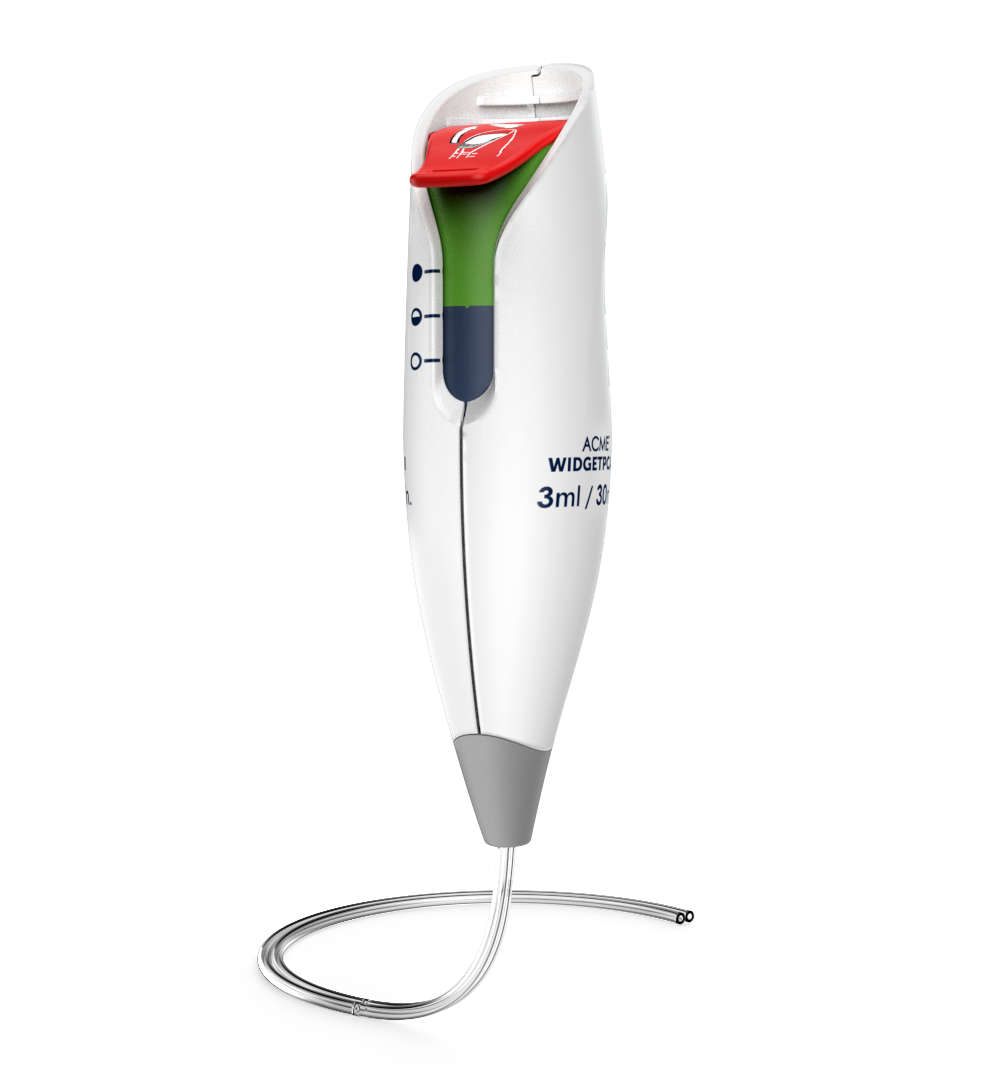

Ergonomic, modern PCA controller
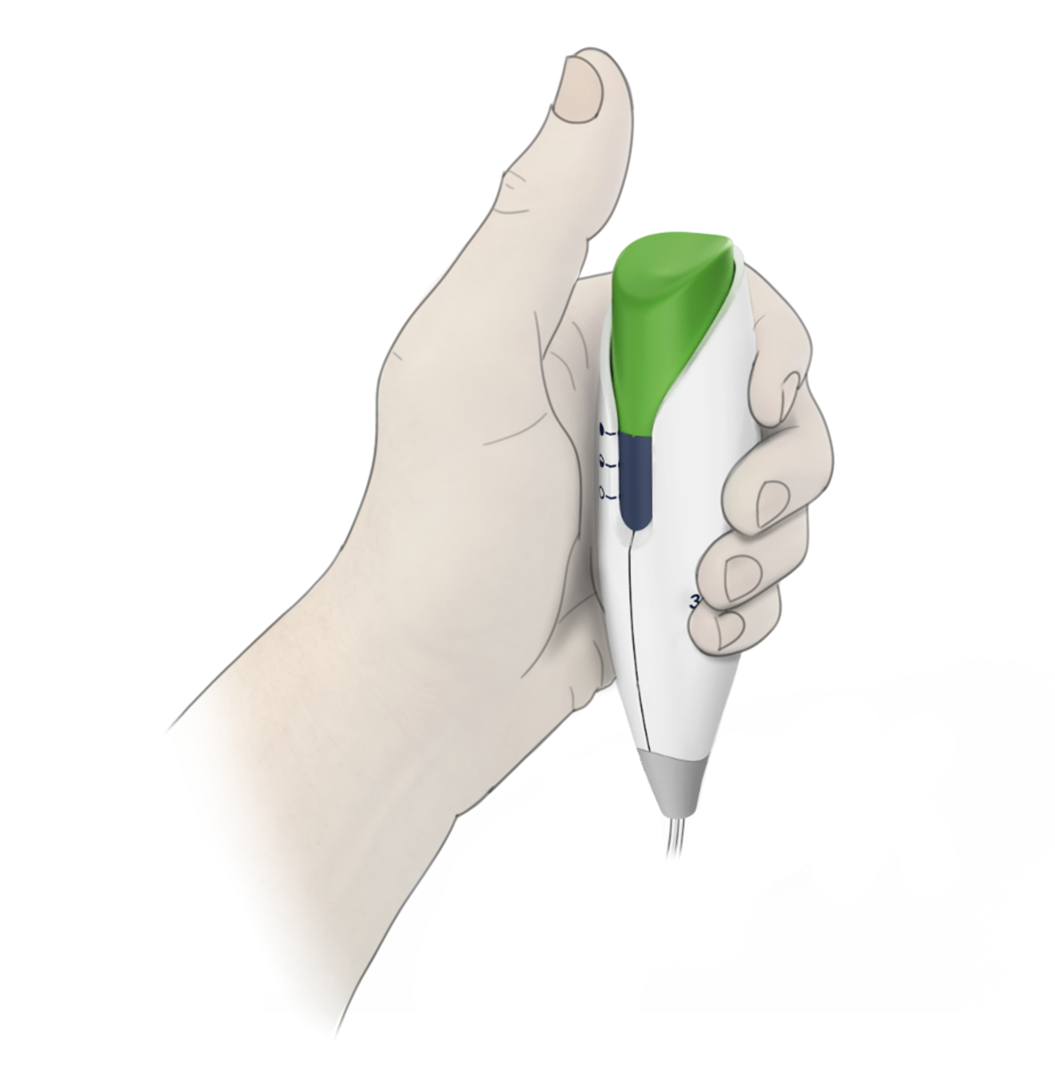
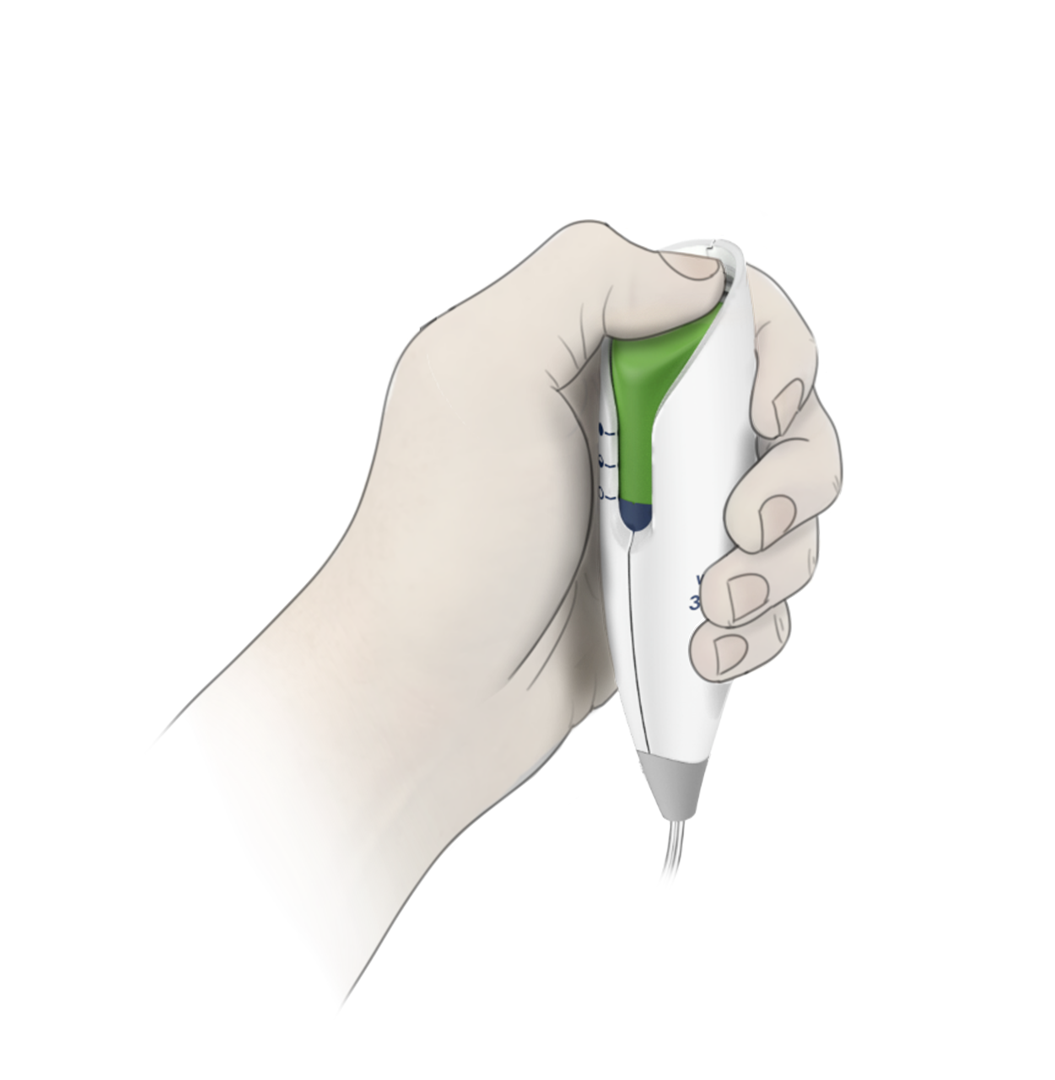
Intuitive UI, low use-error risk
Micro-fluidic tech for reliability & cost
Halyard Health, (now Avanos Medical) contracted BlackHägen Design to develop a new, low-cost patient PCA controller for their ON-Q* Pain Relief System. The new controller provides on-demand bolus delivery for about 70% of the outgoing device, with increased reliability while also reducing use-error and maximizing ergonomics. The new system will continue Avanos Medical’s non-opioid approach to pain management.
The team initiated the project by conducting contextual inquiry on the current ON-Q* system as well as competitive devices to discover unmet user needs. The resulting VOC data was analyzed to refine marketing requirements and inform the design process. In parallel, the systems design team developed a new micro-fluidic technology to substantially reduce device cost and improve reliability.
BH and Halyard engineers collaborated on developing and testing the new fluidic design while the development team created the modern and ergonomic enclosure and intuitive user interface. The resulting commercial design was honed and optimized through a robust international usability test regimen, and met all critical needs for simplicity, low-cost, reliability and use-safety. The Avanos PCA controller for the ON-Q* with Bolus Pump Infusion Device is sleek, modern and friendly, echoing a strong corporate brand identity and class-leading functionality.
The ON-Q* with Bolus Pump Infusion Device will be commercially launched in the future.
*FDA Approved for the US April 3, 2019
The team initiated the project by conducting contextual inquiry on the current ON-Q* system as well as competitive devices to discover unmet user needs. The resulting VOC data was analyzed to refine marketing requirements and inform the design process. In parallel, the systems design team developed a new micro-fluidic technology to substantially reduce device cost and improve reliability.
BH and Halyard engineers collaborated on developing and testing the new fluidic design while the development team created the modern and ergonomic enclosure and intuitive user interface. The resulting commercial design was honed and optimized through a robust international usability test regimen, and met all critical needs for simplicity, low-cost, reliability and use-safety. The Avanos PCA controller for the ON-Q* with Bolus Pump Infusion Device is sleek, modern and friendly, echoing a strong corporate brand identity and class-leading functionality.
The ON-Q* with Bolus Pump Infusion Device will be commercially launched in the future.
*FDA Approved for the US April 3, 2019





Clear Filters