SAIL
Radial Access Core Products Discovery Research informs Product Development
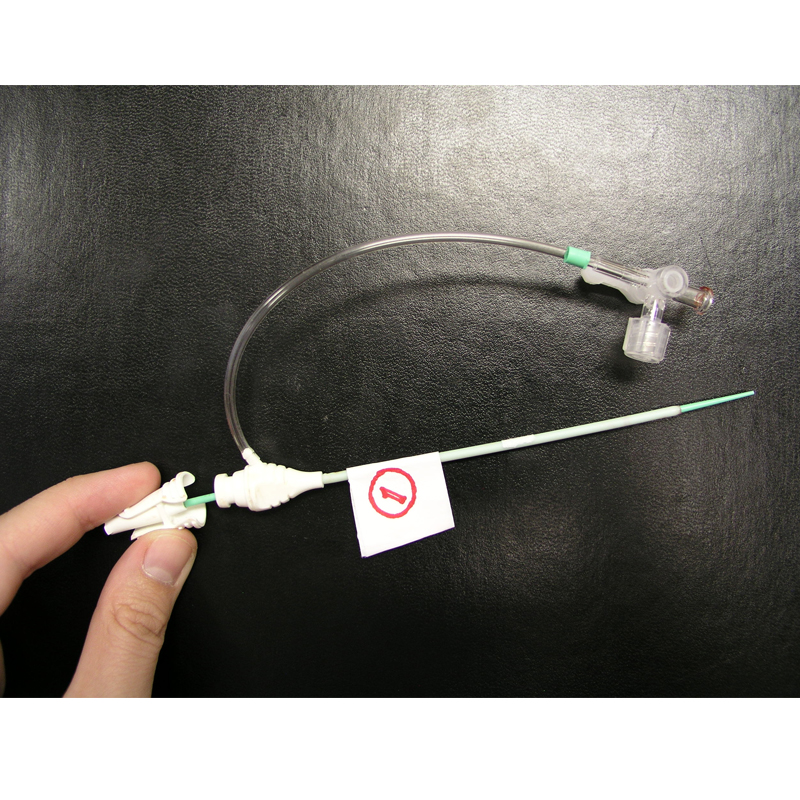
J&J Cordis approached BH to conduct global research and provide product development services to characterize Best-In-Class (BIC) procedures and devices related to Radial-artery Access (RA) Interventional Cardiology procedures. A relatively new procedure, RA was being widely practiced in Japan and gaining popularity in Europe due to the safer, less invasive access site in the wrist rather than the traditional groin access through the Femoral Artery which required a 1-2 day hospital stay after closure to ensure hemostasis, resulting in a substantial cost savings over femoral access, less patient discomfort and inconvenience.
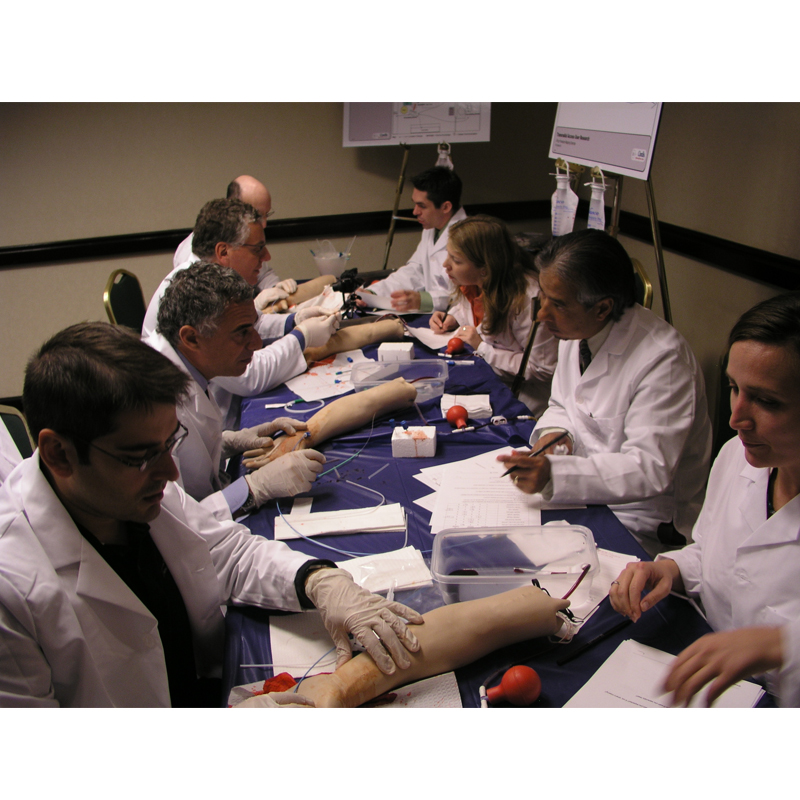
At the time, all RA devices had merely been adapted from femoral instruments and J&J was interested to understand how the RA procedure may be improved by designing core devices that were optimized for RA. BH's charter was to conduct global contextual inquiry (CI) wherever RA was being widely practiced so that appropriate design inputs could be collected to inform the design process. Clinical sites in Japan, Europe and the USA were included in an extensive study, and BIC devices identified for characterization as predicates. The CI study also mapped novel procedural and workflow innovations that would help the design team create class-leading devices. BH also conducted double-blind studies with physicians using simulators to determine if there was marketing bias to perceived device performance.
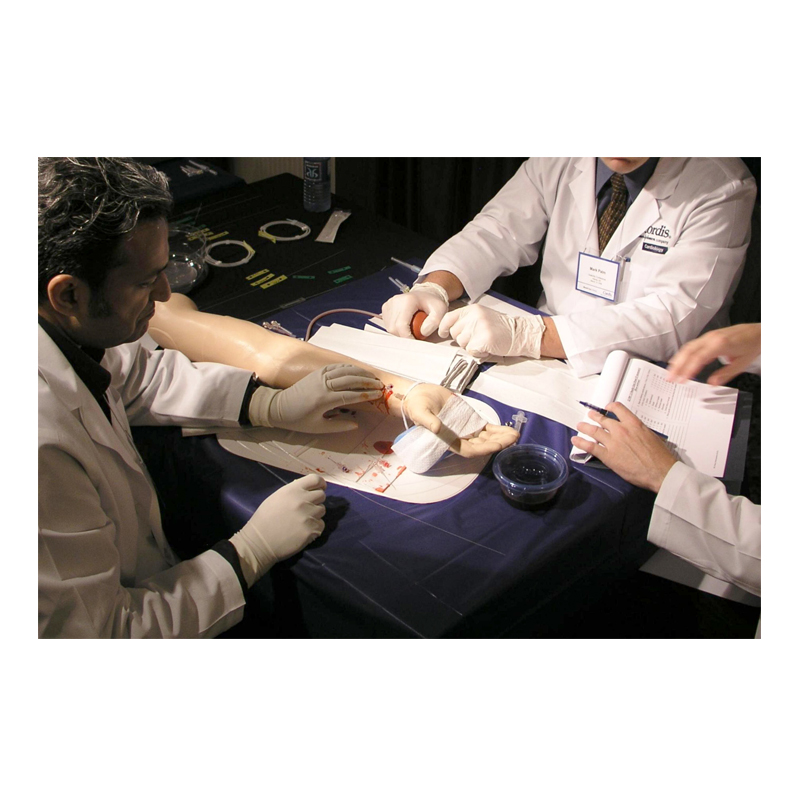
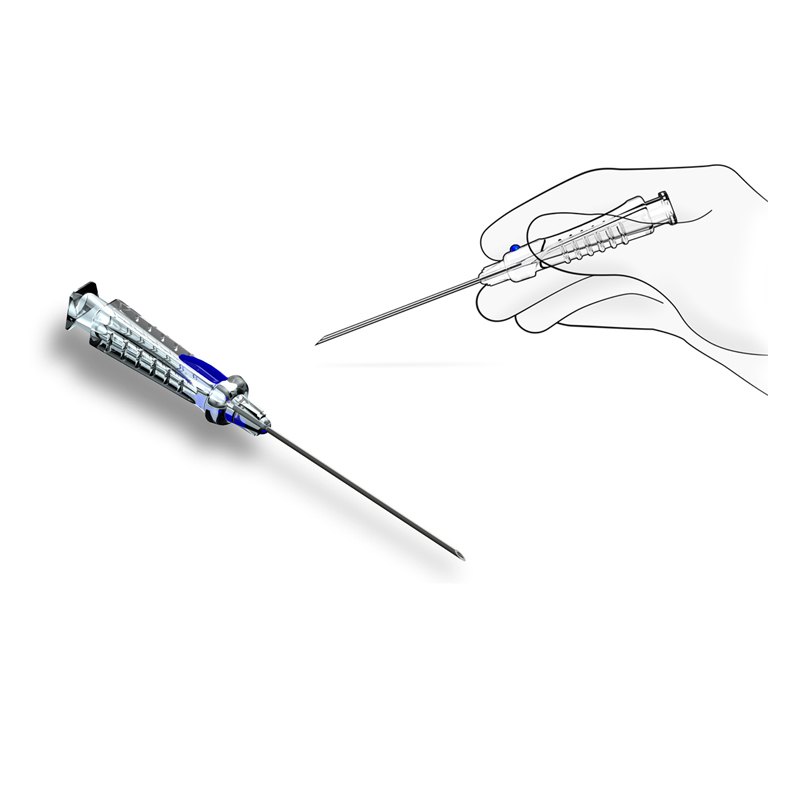
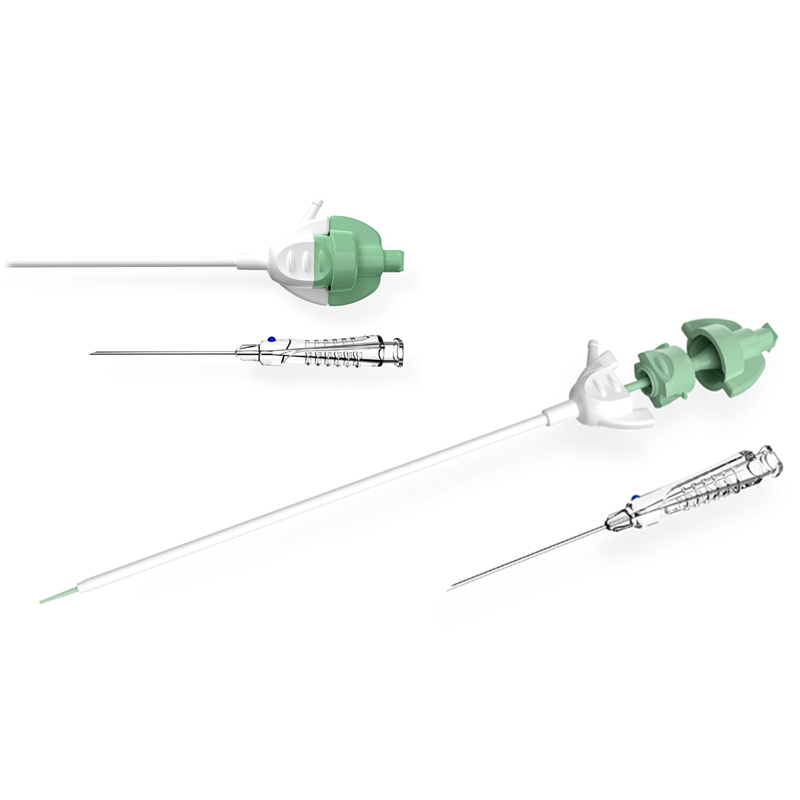
BH conducted an innovation summit (Charrette) with Cordis teams to disseminate all Discovery research output and to collect viable concepts for new RA-dedicated devices. Study models representing the best concepts were then created and tested across critical markets in the USA, Japan and Europe, with feedback supporting the down-selection of initiatives for production. This included a new Access Needle, Introducer/Dilator and modular RA Kit.
BH was then contracted to develop the Needle and Introducer/Dilator devices, working closely with Cordis Marketing/R&D teams and contract manufacturer, to finalize and launch the new RA devices. Additional usability studies were conducted to confirm the final designs, including design validation testing.
Duration- 24 months